To close critical hygiene protocol compliance gaps, healthcare facilities need a practical and reliable way to monitor staff performance. Catalyst embraced the opportunity to develop a next-generation solution. In collaboration with our IoT partner, MESH Systems, Catalyst designed, engineered, tooled, and manufactured the housing and support systems for a real-time monitoring device. Catalyst accelerated the project to deliver a fully functional and scalable product, ready for deployment in clinical environments, to strengthen compliance and protect patient safety.
The Challenge: Designing for Function, Compliance, and Serviceability
Development of the enclosure included facilitating the seamless operation of motion sensors, Bluetooth and other radio communications to confirm compliance of protocol. The viability of the IoT technology depended on a housing design that was robust, functional, and easy to install, use, and service. Key challenges included creating a serviceable battery housing for flexible installation options and validating the design through OEM and regulatory processes.
Bringing the Monitoring Solution to Life
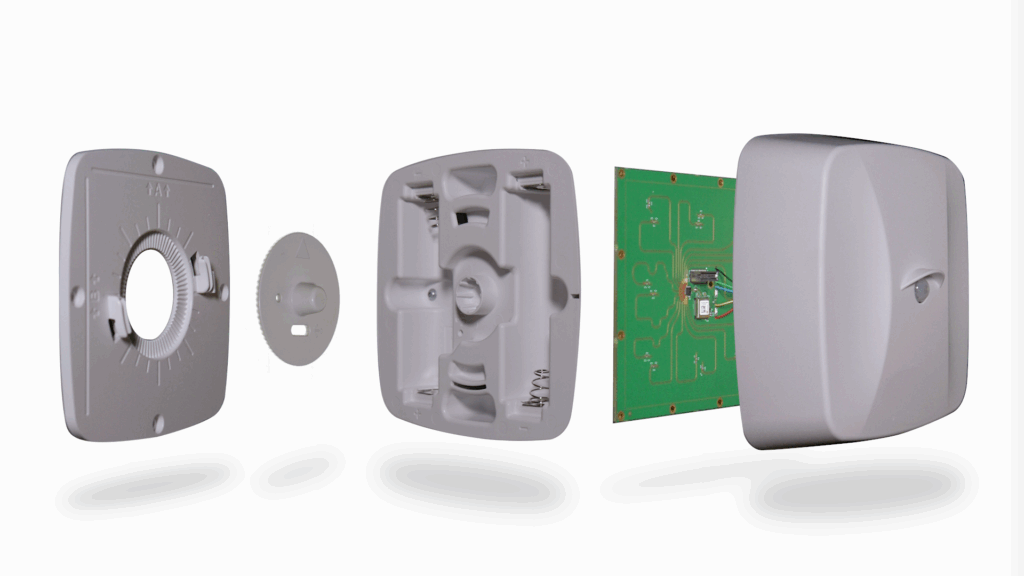
Catalyst provided full-service product development support, bridging early electronic concepts into a manufacturable, hospital-ready device.
Key contributions included:
- Engineering & Design: The Catalyst design and engineering team created manufacturable design concepts and CAD models that integrate the design language of the client’s existing products, while focusing on functionality and adaptability for diverse clinical installation scenarios.
- Battery System Integration: Challenged with a requirement of uninterrupted service for over two years, Catalyst designed a modular, serviceable housing for sufficient battery power with user-friendly access.
- Tooling & Validation: Catalyst designed and built plastic injection molds for all enclosure components in-house, collaborating with the OEM for approvals. The molds were then used in our plastic injection presses to produce an initial run of molded components that were validated through functional testing.
- Pilot Production: Catalyst associates produced one hundred functional pilot units to eliminate any hardware/software integration concerns and establish an efficient protocol for mass assembly before final production.
- Scaled Manufacturing: Catalyst assembled and delivered 1,000 production units with custom packaging, ensuring secure and compliant shipment. As an added benefit to our client, Catalyst maintains the molds on site with the ability to quickly scale production as demand requires.
Improved Compliance, Safer Patients, and Scalable Production
Our product realization process enabled the client to quickly bring an IoT monitoring solution to market, while empowering healthcare providers with actionable data to improve compliance and reduce infection risks. Catalyst’s end-to-end involvement ensured the device was reliable, maintainable, and ready for deployment in critical healthcare environments.
The partnership continues as demand grows, with Catalyst supporting new production runs and collaborative sourcing of electronic components.
By combining IoT innovation from MESH with Catalyst’s expertise in medical device design and manufacturing, the client successfully launched a product that empowers hospitals to meet compliance goals and improve patient safety outcomes.
From infection prevention to life-saving innovation, Catalyst helps transform ideas into hospital-ready devices. Let’s collaborate to bring your next solution to market—faster, smarter, and with confidence.